
GDI technology, or to give it its proper name, Gasoline Direct Injection, has been with us for several years, and while not all manufacturers use it on all their models, direct fuel injection will soon become the norm rather than the exception on all petrol engines sold in all automotive markets. Like many innovations in the automotive world, direct petrol injection is more the result of ever more stringent exhaust emissions regulations than it is of a desire on the part of car manufacturers to improve the general efficiency of petrol engines. Nonetheless, as it turned out, one happy consequence of direct injection engines is that they not only use substantially less fuel than port injection engines- they also make more power, while emitting fewer emissions than comparable port injection engines do, at the same time.
While this might sound like a win-win situation for the car-buying public, nothing is ever free. There is always a price to pay, and in the case of direct fuel injection, this price is the fact that direct injection engines are more efficient than port injection engines only for a limited time, but to restore a direct injection engines’ efficiency can sometimes be eye-wateringly expensive. Thus, in this article, we will take a new look at direct petrol injection engines in terms of how a natural consequence of their design affects their efficiency. Let us start with stating-

Image source: https://www.vehicleservicepros.com/service-repair/diagnostics-and-drivability/article/21239948/carbon-calamities
This image shows a relatively light accumulation of carbon on a pair of intake valves from the same engine, but before we get to specifics, it must be stated that this kind of problem is not confined to the direct injection engine of only some manufacturers. All direct injection engines from all manufacturers have the problem of extreme carbon build-up on their valves, although the rate of carbon build-up is faster (or slower) on some engines than on others.
In the case of the example above, the amount of carbon on the valves is relatively small but the important thing about this example is that the carbon on these valves took only 20 000km of mostly highway driving to reach this level. More importantly, though, this level of carbon accumulation was serious enough on this particular vehicle to produce serious misfires when the engine was running at idling speed, which is the most common sign on all direct injection engines that the amount of carbon on the valves is reaching critical levels, which begs the question of-
The principal (but not the only) cause involves the differences between port injection and direct injection. Put simply, with port injection the fuel is delivered to a point behind the intake valve(s), so the vapourised fuel flows over the valve(s) as it a) mixes with the inrushing air, and b) as it flows into the cylinder(s). As a practical matter then, the vaporized fuel continually removes incipient carbon deposits from the valves as it flows past and over the valves.
By way of contrast, direct injection delivers the vapourised fuel directly into the cylinders, hence the term "direct injection". While this strategy greatly improves the combustion process, it only works as intended when the valves are clean and free of carbon deposits, so let us look at how the carbon forms-
It is tempting to think that direct injection engines are only port injection engines on which the fuel injectors had been moved to a point where they inject fuel directly into the cylinders, but this would be wrong. In practice, direct injection on petrol engines represents a paradigm shift in terms of not only piston and combustion chamber design, but also in terms of fuel delivery and airflow dynamics through the intake manifold.
Limited space precludes a comprehensive discussion of these aspects of fuel management, but suffice it to say that in a new direct injection engine, the air/fuel mixture resembles a “ball” of gas in which the air largely envelops the fuel to prevent fuel droplets from condensing onto the cylinder walls. However, while the centre of the ball of gas is forced towards a point close to the spark plug electrode as a function of the piston crown’s design, the air/fuel mixture is not homogenous. In practice, the concentration of fuel relative to air is highest towards the centre of the ball, and in some engines, the air to fuel ratio towards the edge of the ball can be as lean as 65:1.
While this generally produces almost perfect combustion at idling speed, the process is further refined on some engines that deliver several injections of fuel combined with several ignition sparks per injection/combustion cycle. We need not delve into complexities of these refinements here, beyond saying that the intake systems on direct injection engines are specifically designed to reduce, if not eliminate, turbulence and bouncing pressure waves in the intake runners, which if they occur, have serious negative effects on how effectively the air envelops the fuel in the cylinders before combustion.
While all of the above may sound like a digression, it is important to understand these aspects of direct injection if we are to understand how carbon deposits form on valves, so let us discuss this process one step at a time as it happens and progresses in a new direct injection engine-
The above bullet points represent the short version of how carbon forms on the valves of direct injection engines, but as stated elsewhere, the first sign that carbon deposits on valves have reached critical levels is when the engine begins to exhibit misfires at idling speed, or in serious cases at speeds just above idling. Note that on most direct injection engines, the misfires are very pronounced when the engine is cold, and abate somewhat as the engine warms up.
Also, note that this point varies between engines; in some cases, misfires can start to happen from about 20 000km, while in others, the first symptoms might only appear at around the 30 000km to 35 000 mark. At this point, a customer might ask you about ways of resolving the misfires, but it is also at this point where it becomes easy to fall down the chemical cleaning vs. walnut blasting rabbit hole, because-
We need not delve into the chemical composition of carbon deposits here, beyond saying that the hardness, porosity, and resistance to chemical attack are all functions of the heat and pressure under which the deposit formed. For instance, since intake valves are always cooler than exhaust valves, carbon on the intake valves is always softer and more porous than carbon that forms on extremely hot exhaust valves. As a result, it is usually easier to remove carbon deposits from intake valves than it is to remove carbon deposits from exhaust valves.
Nonetheless, the actual chemical composition of carbon depends on more than heat and temperature. Limited space again precludes a comprehensive discussion of the topic, but suffice to say that the two most important factors that determine the composition of carbon deposits are fuel quality/grade and the type and formulation of the engine oil.
The problem with oil is that even though branded oils (usually) comply with the required technical specifications set out by car manufacturers, oil formulators go about achieving compliance in different ways. These differences involve not only the relative concentrations of additives like detergents, friction modifiers, anti-foaming agents, and corrosion inhibitors each oil formulator uses but also the actual chemicals different oil formulators use to do the same thing.
What this means in practice is that while all branded engine oil formulations provide adequate lubrication to direct injection engines, the additives in different oil formulations react differently to heat, and contact with oxygen and combustion waste products in different engines. Put differently, this means that some volatile constituents of one branded oil formulation may evaporate at higher or lower rates than similar constituents in some or all other branded oil formulations.
This, in turn, means that growing carbon deposits in some engines are not only “fed” with higher or lower hydrocarbon loads than carbon deposits in other engines, but also that the types of hydrocarbons in the growing carbon deposits vary according to the additives used in the engine oil. Moreover, this does not only affect the rate of growth, structure, and porosity of any given carbon deposit but also any given carbon deposit’s resistance to attack by (some) chemical agents that are (supposedly) designed to remove carbon deposits from valves, which begs this question-
Broadly speaking, the removal of carbon deposits from the valves of direct injection engines is possible but the success (or otherwise) of chemical cleaning processes depend on a few critically important factors, these factors being-
Of the points listed above, the last one is the most important by far, since even a purpose-made chemical agent won't remove carbon deposits from valves if it is not applied correctly or effectively. Consider the image below-
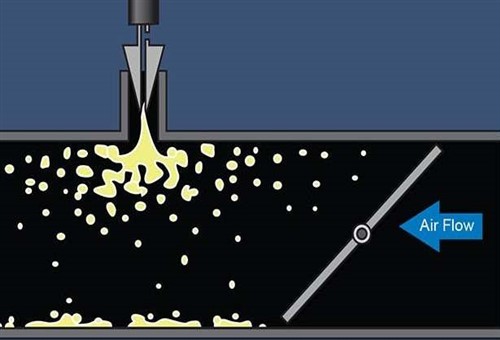
Image source: https://www.vehicleservicepros.com/sites/www.searchautoparts.com/files/images/dr-Figure%206.jpg
For many years, the industry-standard in applying chemical carbon removers has been to use the engine vacuum to "suck" a liquid chemical out of a container, as shown in this graphic that represents engine vacuum sucking a liquid chemical into the inlet tract through an orifice such as a vacuum hose attachment point.
The thinking behind this method had always been that a) the turbulence in the inlet tract after the throttle plate will break up the liquid into small droplets and b) that the force of the airflow will then carry the small droplets towards the valves. However, in practice, the droplets are almost always too big to be broken up by the airflow in the inlet tract, and what most often happened was that the liquid chemical amalgamated into small pools in "dead spots" in the inlet tract where the airflow was the least forceful. As might be expected, this method delivered chemicals to the valves either in drips and drabs or in large "blobs" that had a limited effect on carbon deposits – if they had any effect at all. Now consider the image below-
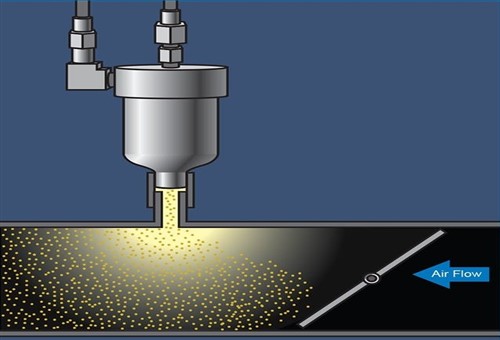
Image source: https://www.vehicleservicepros.com/sites/www.searchautoparts.com/files/images/dr-Figure%207.jpg
This graphic represents an altogether more efficient and effective method of introducing chemicals into the engine. In this type of system, the chemical is highly pressurised and injected into the inlet tract (after the throttle plate) in a fine mist that is easily carried toward the valves by the airflow in the inlet tract.
The greatest advantage of this system is that the cleaning agent reaches the valves while it is in the form of a fine mist that covers the total surface of the inlet port. Thus, the entire inlet port and the valves are continually bathed in the mist, which ensures that the cleaning agent acts continually on the entire carbon-covered area. Consider the before and after images of a pair of valves below-

Image source: https://www.vehicleservicepros.com/service-repair/diagnostics-and-drivability/article/21239948/carbon-calamities
This image shows some heavy carbon build-up on the intake valve of a late-model Volvo GDI engine after only 22 000km of driving. In this example, the engine has forced induction, and the engines’ general oil consumption averaged around 350ml per 1000km of mostly highway driving. When the vehicle began to misfire at idling speed, the customer presented the vehicle to a dealership to investigate and resolve the problem. The dealership duly investigated, and when they found a severe carbon build-up on the valves, they decided to subject the valves to a chemical cleaning process. The result (after three applications of a leading chemical cleaning agent) is shown below-

Image source: https://www.vehicleservicepros.com/service-repair/diagnostics-and-drivability/article/21239948/carbon-calamities
While this is a remarkable result, the repeated application of the cleaning agent did not resolve the misfiring problem at idling speed, although the misfires were not quite as severe as before the cleaning process. Nonetheless, when the customer presented the vehicle to us to investigate the issue again, we found that while the chemical agent did remove most of the carbon from the valve, it removed almost no carbon from the walls of the intake port. Consider the two red arrows in the above image; these indicate lumpy carbon deposits on the intake port's walls that partially obstruct the airflow around the valve head, which we confirmed by installing a pressure transducer in the cylinder shown here, and scoping the pressure in the cylinder while cranking the engine. Here is what we saw-
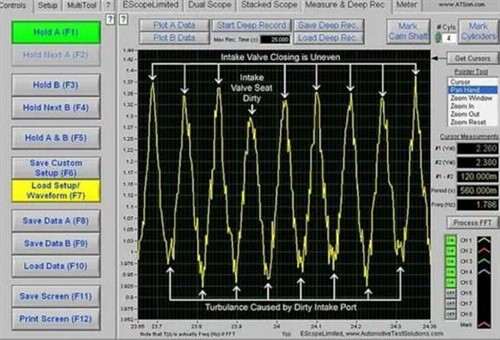
Image source: https://www.vehicleservicepros.com/service-repair/diagnostics-and-drivability/article/21239948/carbon-calamities
This waveform clearly shows the turbulence caused by the disrupted airflow around this particular intake valve, and in terms of practicalities, this result demonstrated two things yet again. The first is that chemical cleaning treatments do not always work as expected, and the second is that if multiple applications of a purpose-designed chemical do not remove all carbon deposits in an engine, subsequent applications of the same chemical will also not remove the carbon deposits that remain after the first applications.
It is perhaps worth mentioning that the treatment that was applied to the vehicle in this example consisted of three distinctly different chemicals, each of which is designed to attack, dissolve, and remove a different "flavour" of carbon. As a general rule, the outer layers of a thick carbon deposit are usually soft, tarry, and very porous, which makes them relatively easy to dissolve and remove. The middle layers are usually more compact, denser, and harder than the outer layers, which is why most advanced chemical treatments consist of different chemicals that are designed to attack different types of carbon. Moreover, the layers of carbon that directly overlay metal surfaces are usually very dense, very hard, and highly resistant to attack by even the most advanced chemical agents.
WARNING: While chemical treatments can be reasonably effective, it should be noted that many chemical cocktails in common use in the automotive industry to remove carbon deposits contain N-methyl-2-pyrrolidne (aka NMP), which is known to cause testicular cancer. Therefore, we recommend that you avoid using chemical treatments that contain NMP in any concentration since there are many less harmful chemicals available that are as effective as NMP in removing carbon deposits from GDI engines.
In this case, the customer had two options; we could continue to pump chemicals through the engine to see if we could dissolve the remaining carbon deposits, or we could remove the intake manifold to remove the carbon mechanically by-

Image source: https://www.vehicleservicepros.com/service-repair/diagnostics-and-drivability/article/21239948/carbon-calamities
In its simplest form, walnut blasting resembles sandblasting, but instead of sand, the system uses coarsely- powdered walnut shells as an abrasive. As shown here, the abrasive is blasted directly into the intake port (while both valves are closed!) through the thin hose, and a vacuum cleaner immediately recovers the (now) pulverised walnut shells to suppress dust.
As a practical matter, this writer has yet to encounter carbon deposits in GDI engines that walnut blasting cannot remove, but despite its advantages and effectiveness, this method also has serious drawbacks. For example, it is sometimes impossible to remove the intake manifold without removing the engine from the vehicle, which few customers allow. While it is possible to perform walnut blasting on such engines by removing the cylinder head(s), most customers are equally reluctant to allow this very expensive workaround.
Nonetheless, where walnut blasting is possible without customers incurring additional labour costs, this method remains the most effective method of removing carbon deposits from the valves of GDI engines. As it happened, the owner of the Volvo we were working on declined the offer of a walnut blasting to clean out his engine because he did not have the budget to remove the intake manifold on his engine, which leaves us with this-
The secret to selling chemical valve cleaning treatments is to manage the customer's expectations. We would suggest that it is far better to tell the customer upfront that while chemical treatments do work, they do not always work as expected and, sometimes, they do not work at all than it is to explain to an unhappy customer that chemical treatments do not come with guarantees when a treatment fails.
Whatever approach we take in selling valve cleaning treatments, though, the fact is that GDI technology will be with us for a long time to come, as will the issues that come with carbon build-up in GDI engines. Thus, if this writer could offer some (unsolicited) advice it would be this; educate your customers that have DGI engines on the importance of sticking to their servicing schedules, sticking to one brand of oil, and not ignoring misfires or even just a bit of roughness when their engines are running at idle.
Carbon build-up on valves in GDI engines is unavoidable, but by at least doing regular servicing, and paying proper attention to the early warning signs of carbon building up, they could likely benefit from proper chemical cleaning treatments. As a rule, these are a lot cheaper than, say, you removing the engines from their cars just so you could access the intake ports to use your walnut blasting equipment.