
In Part 1 of this series of two articles, we discussed the basic operating principles of flooded lead-acid batteries. In Part 2, we the most important factors that can and do influence some battery test results.
These factors are the facts that flooded lead acid batteries suffer from a) a phenomenon known as self-discharge and b) the misconception that lead-acid batteries tend to develop charge memories. While self-discharge does occur in lead-acid batteries, lead-acid batteries do not develop charge memories, even though this misconception is still firmly rooted in the minds of many car owners and mechanics, alike.
Nonetheless, both self-discharge, which does happen, and charge memories, which do not, can and sometimes do produce inconclusive and/or misleading test results, depending of course, on who does the testing, how the testing is done, what equipment is used to test the battery, and how the test results are interpreted.
So, having said the above, let us start with this question-
Although the process by which all lead-acid batteries discharge themselves is a natural consequence of all lead-acid battery chemistries, there is a lot of confusion about what constitutes a "normal" self-discharge rate, and what constitutes abnormal self-discharge rates caused by various kinds of defects in a battery.
Since both natural and abnormal self-discharge processes manifest in the same way, i.e., the battery runs down over time even when there are no loads on it, it is sometimes very difficult to distinguish between natural, and abnormal self-discharge processes if you do not take the rate of discharge over set periods into account. Consider the graph below-
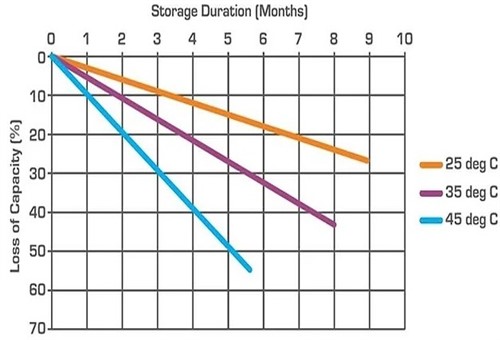
This graph shows how much capacity flooded lead-acid batteries can potentially lose based on storage periods and ambient temperatures. Note that when batteries are stored at 25 degrees Celsius, they lose less than 30 per cent of their capacity over periods of as long as nine months, while the same batteries can lose more than 50 per cent of their capacity in less than six months when they are stored at ambient temperatures of 45 degrees Celsius. Note, though, that although self-discharge rates are also influenced by factors like a battery’s quality, exposure to direct sunlight and/or high relative humidity values, as well as large temperature fluctuations during storage, the information in the above graph is fairly representative* of the self-discharge rates of all high-quality lead-acid batteries at the temperatures shown in the graph.
* Note that as a rule of thumb, the self-discharge rates of AGM (Absorbed Glass Mat) batteries are about 50 per cent lower than those of flooded lead-acid batteries at comparable temperatures, and under similar storage conditions.
We need not delve into the fine details of the chemical processes that cause lead-acid batteries to self-discharge here, beyond saying that since lead-acid battery chemistries are not ideal, all chemical reactions and/or processes in all lead-acid batteries can flow or proceed in both directions.
Put differently, this means that a battery’s open circuit voltage can increase briefly when a charger is removed because the process that charges a battery sustains itself for a short time after an input/charging current is removed. Similarly, both the presence of sulphate crystals on a battery’s plates, and the presence of impurities such as dissolved lead dioxide in the electrolyte can, and does, imitate the effects that a load has on the overall chemistry of a battery, thus causing the battery to discharge itself.
From a battery diagnostic perspective, however, the very long periods it takes for a battery that is in a good condition to discharge itself is proof that there is a minimal/acceptable amount of sulphate and impurities in the battery. By way of contrast, when a lead-acid battery discharges itself over a few days, or even a few hours when there are no loads on it, it is safe to say that such a battery is a) sulphated*, and/or b) that the electrolyte in such a battery is saturated with dissolved lead compounds. These conditions greatly accelerate the self-discharge process because the impurities effectively short-circuit the negative and positive plates.
*The presence of excessive amounts of sulphate crystals on the battery’s plates, or sometimes, the presence of accumulations of large sulphate crystals that are lodged between the plates.
Thus, based on the above, the guiding diagnostic principles are-
It should also be noted that since the normal use of most vehicles does not allow lead-acid batteries sufficient time to self-discharge naturally to meaningful degrees, high self-discharge rates are invariably the result of excessive sulphation or other defects in the battery, which brings us to-
We can save a lot of time by saying that lead-acid batteries do not develop charge memories, aka memory effects, simply because their chemistries simply do not allow or support this phenomenon to occur; ergo, charge memories in lead-acid batteries are fiction.
However, this begs the questions of a) what is a charge memory, exactly and b) if lead-acid batteries do not develop charge memories, why is there so much talk/debate/disagreement about it in almost any online automotive-related discussion forum one cares to visit? These are two very good questions, so let us start with-
There are many ways to define the term "charge memory", or the memory effect of batteries, but the easiest way to do is to say that this condition refers to a battery's inability to a) accept a charge when its open circuit voltage is above a certain point, and b) to produce electrical energy under load when its open circuit voltage is below a certain point.
However, this condition only affected nickel-cadmium and some nickel-metal-hydride batteries from about 40 years ago. The basic mechanism involved unwanted but unavoidable, reactions in the chemical makeup of these batteries, but for the most part, these unwanted reactions could be reversed or minimized by proper battery maintenance procedures.
In practice, the chemistry of lead-acid batteries does not allow the development of charge memories, although some defects or conditions could sometimes mimic the effects of a charge memory in some lead-acid batteries, which brings us to-
As with many other aspects of car care and maintenance, there is a lot of misinformation, disinformation, ignorance, confusion, and deliberate obfuscation not only in many online discussion forums that cater to the general public but also in a large number of online resources that purport to provide expert advice about car maintenance matters to audiences of different skill levels.
The problem with these kinds of online resources is that many members or participants often don’t know enough about topics under discussion, such as, for instance, charge memories in lead-acid batteries, to know or realise that the information they are reading is often fragmentary, incomplete, misleading, unverified, confusing, or downright wrong. Therefore, as a result of not knowing what is true or not, many members of such resources have no problems at all with a) accepting what they are reading as the gospel truth, and b) spreading unreliable, incorrect, and/or misleading information to yet more dubious online resources.
At the risk of being uncharitable, many discussions in resources of the kind described above are either initiated or dominated by people who like to play One-eyed King in the Land of the Blind, which explains why so much unreliable information is repeated almost word-for-word on so many online resources.
Examples of this abound on the Internet, but the point is this: if you are looking for reliable information on automotive battery maintenance, who would you rather believe- the word of a self-proclaimed expert on a random discussion forum, the real-world experience of an experienced technician, or the verifiable, peer-reviewed results of academic research on the topic of proper battery maintenance? This writer always goes with one or both of the last two options.
We mentioned ignorance about some topics earlier, and while we cannot reasonably expect all car owners to be experts on the topic of battery maintenance, none of us, as mechanics and technicians, can have an excuse to confuse even the common symptoms of defective or failing lead-acid batteries with those of a charge memory, which is a condition that cannot occur in lead-acid batteries.
The above is saying a lot, especially if you are just starting your career in the car repair industry, but learning to recognise the most common symptoms of failing lead-acid batteries is not as hard as you might think. In fact, with a few exceptions, problems like slow engine cranking speed, battery capacity running out after only a few cranking revolutions, or the engine not starting during cranking but starting the moment that you release the key, have much more to do with how a battery is used than with actual battery defects or faults.
Being aware of this fact will help you diagnose many kinds of electrical issues on modern cars much quicker, so let us look at some battery issues that do not involve actual defects in flooded lead-acid batteries, starting with-
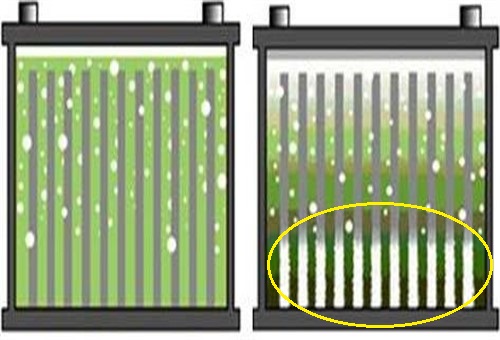
The graphic shown here shows two flooded lead-acid batteries; the electrolyte in the battery on the left is in a normal state, while the electrolyte in the battery on the right is severely stratified, as shown by the yellow oval, which represents the area in which the concentration of sulphuric acid in the electrolyte is highest.
In simple terms, “stratification” refers to a condition in which the sulphuric acid in the electrolyte separates out of the solution, to form different strata, or layers in the battery, each of which contains a progressively higher concentration of acid towards the bottom of the battery. As a practical matter, this condition deprives the upper half of the plates of acid, which means that the upper half of the battery generates much less electricity than the bottom half of the battery.
In practice, this condition is produced when a flooded lead-acid battery is used for extended periods while its state of charge is below about 80 per cent, never receives a full charge, and is only ever discharged shallowly, as opposed the being discharged deeply. These conditions typically obtain in luxury vehicles that are only used for short trips that do not give the alternator sufficient time to raise the battery's state of charge to above 80 per cent, which brings us to-
The symptoms of acid stratification
Since the heavier concentration of sulfuric acid in the lower half of the battery raises the open circuit voltage of the battery, a stratified battery might appear to be fully charged. However, the CCA (Cold Cranking Amps) of a stratified battery will be drastically reduced as a result of the stratification, which means that the engine may crank very slowly, or not at all, even though the battery appears to be fully charged.
This condition is not a battery defect as such, and can almost always be reversed simply by giving the battery a vigorous shake-up or laying it on its side to redistribute the acid evenly throughout the electrolyte solution. One other method of reversing acid stratification is to raise the battery’s state of charge to 16 volts for a maximum of two hours to allow the action of moving gas bubbles to redistribute the acid throughout the electrolyte solution.
Note, though, that while the above methods usually restore a stratified battery’s capacity, they may not work in extreme or recurring cases. In such cases, the most reliable remedy is to replace the flooded lead-acid battery with an AGM (Absorbed Glass Mat) battery, which is much less prone to both acid stratification and sulphation caused by persistent low states of charge.
The main reason why lead-acid batteries take as long to charge as they do is the fact that the lead-aid chemistry cannot convert lead sulphate into lead and lead dioxide quickly during charging. This conversion process is the basis of the charging process, and sometimes, the bulk of the charging process occurs on the surfaces of the plates only, if the battery is connected to a “fast charger), hence the term, “surface charge”.
As a practical matter, a surface charge raises an affected battery’s open circuit voltage artificially, which could have serious implications for some repair procedures, such as module programming. Note that t is standard practice to charge a vehicle’s battery before module programming, but if a surface charge is present, the dissipation of the surface charge could cause the battery’s voltage to drop suddenly, which if it happens, could cause a programming event to abort.
While connecting a clean and stable DC power supply to the battery before module programming procedures is also standard practice, doing this will usually not dissipate or remove a surface charge, which could raise the battery’s apparent state of charge to above recommended levels for module programming. Thus, the best way to ensure a surface charge is not present after charging a battery is to let the battery rest for about six hours or to remove about one per cent of the battery's capacity by turning the headlights on, and keeping them on without the engine running for about 30 minutes or so- depending on the battery’s rated capacity.
Note that surface charges are not battery defects as such, but the result of some battery charging processes. Also, like acid stratification, a surface charge is completely reversible but despite this, many batteries are unnecessarily condemned out of hand because they don't seem to "hold a charge" when the observed or measured state of charge suddenly decreases as the surface charge dissipates, which leaves us with-
In a follow-up article, we will discuss a few battery tests that are not only easy to do with inexpensive equipment but also produce usable results about a lead-acid battery’s overall condition in terms of both its state of charge and its state of health.
However, since a battery's high state of charge does not always translate into a good state of health, the cause of the disconnect between the two states is sometimes difficult to diagnose or identify unless one uses highly advanced equipment such as, among others, capacitance and resistance testers that make extensive use of artificial intelligence to infer a battery’s condition.
Therefore, based on the fact that this type of equipment is frequently out of the reach of new mechanics, the next article will focus on test equipment you may already own or could acquire relatively cheaply.