
In Part 2 of this series of articles, we discussed some of the principal features of high-performance brake rotors, with particular reference to the brake rotors found on true high-performance vehicles, as opposed to the drilled and/or slotted aftermarket brake rotors many car enthusiasts use to upgrade brake systems on modified passenger vehicles. There are vast and critically important differences between true high-performance brake rotors and their aftermarket lookalikes, and in this final article in this series, we will discuss the most important of these differences in some detail, starting with this question-
The short answer is no. On the one hand, it depends on which segment of the aftermarket is supplying the high-performance brake rotors. On the other hand, it depends on the application you are buying the brake rotors for. For instance, are you buying brake rotors for a street racer that started life as a sedate passenger vehicle that has not undergone major suspension modifications, and still labours under its original weight distribution? On the other hand, are you buying brake rotors for a powerful, high-end sports car whose top speed is limited to 250km/h by its manufacturer?
These are important distinctions because it is easy to forget that the efficiency of brake rotor/friction material combinations is only one factor among many others that make it possible to bring a heavy sports car travelling at a high rate of speed to a stop safely. Some of these factors include brake torque as a function of wheel diameter, the width; tread pattern and composition of the tyres, as well as the vehicle’s suspension geometry, centre of gravity and the overall distribution of weight at each wheel.
In terms of design imperatives, all of these and other factors are programmed into the ABS and other safety features such as traction -, and stability control systems to achieve two primary purposes, these purposes being-
At the risk of putting too fine a point on it, brake rotors are integral components of a high-performance vehicle’s overall design, as opposed to off-the-shelf components that may or may not be effective in bringing the vehicle to a stop safely. Nonetheless, from an engineering perspective, it is not an exaggeration to say that true high-performance vehicles are in some ways built around their brake systems, which relates directly to the question of whether (or not) all high-performance aftermarket brake rotors are suitable replacements for OEM rotors.
We stated in Part 2 of this series of articles that for any aftermarket high-performance brake rotor to qualify as a suitable replacement for an OEM rotor, the aftermarket rotor must be identical to the OEM part in all respects. We mentioned aspects like mass, diameter, thickness, and the arrangement of ventilating holes/slots, among others, and while many aftermarket brake rotors "tick all the right boxes" in these areas, we did not mention the single most important difference between true high-performance OEM brake rotors and generic aftermarket rotors that sometimes resemble OEM parts. This difference is crucially important, and it involves-
It should be noted that in this article, we will concentrate on brake rotors made from grey cast iron, and ignore brake rotors made of various composite materials and metals such as stainless steel and aluminium. These rotors have a limited automotive application due to a) their high cost, and b), the difficulty in limiting the amount of brake dust these types of rotors create.
Note also that the topic of specialised brake rotor coatings is a highly technical one that requires substantially more than a working knowledge of physics, metallurgy, inorganic chemistry, and mechanical engineering to appreciate fully. Therefore, the various methods of applying coatings to brake-rotor surfaces fall outside the scope of this article, but we can do the next best thing, which is to discuss the various and most commonly used materials used in brake rotor coating today.
Having said the above, we must also say that the technical details in the section that follows are drawn from a comprehensive, academic-level literature review in which various aspects of specialised brake rotor coatings are discussed in some detail.
While limited space precludes a comprehensive discussion of the tribological effects of each of the most commonly used coatings that are in use today, we can say that the need to develop effective coatings stems from the need to suppress brake dust, as opposed to stemming from the need to improve the friction coefficient of brake rotors. To put this need into perspective, consider that several studies done in various parts of the world on the effects of high wear rates of automotive friction material on both the environment and human health have shown that brake dust accounts for roughly 55% of non-exhaust automotive emissions, but worse, that some components of brake dust can be carcinogenic.
However, the braking demands of high-performance vehicles are such that holes and slots in brake rotor surfaces have become necessary to improve braking performance, but the downside of holes and slots in brake rotors is that they greatly increase the rate at which both rotors and friction materials wear away, thereby greatly increasing the creation of brake dust.
Therefore, the primary driver behind the development of brake rotor coatings is dust suppression by reducing brake component wear rates, but it turned out that these developments had one happy and largely unintended consequence, which was the discovery that some coatings had the effect of increasing friction coefficients by significant percentages.
The value of this discovery was not lost on manufacturers of brake components, with the result that all major manufacturers embarked on hugely expensive research programs to develop suitable coating materials, and to refine effective methods of applying coatings to ensure measurable improvements in the braking performance of their products. Below are some details of the coating materials that are in common use today-
Metal oxides
The most common oxides include aluminium oxide (Al2O3), titanium oxide (TiO2) and chromium oxide (Cr2O3) due their high resistance to wear and corrosion, as well as their mechanical strength and hardness. These materials are usually applied using a process that “shoots” powdered particles of the metal in a jet of superheated gas or flammable fuel. This process heats both the metal powder and the rotor surface sufficiently for the powdered metal to fuse onto the rotor surface.
Carbides
Typical carbides include tungsten carbide (WC) and chromium carbide (Cr3C2) that is mixed with other metals that could include cobalt (Co), nickel (Ni), chromium (Cr), and iron (Fe), with the proportions of the mixture depending on which carbide predominates in the mix. Most mixtures are applied by one of several high-velocity thermal spray processes that produce coatings with optimal levels of hardness, ductility, and extremely high resistance to wear and corrosion.
Several other exotic combinations of materials that include various percentages of stellite alloys and self-fluxing alloys consisting of nickel, chrome, boron, and silicon bound by an iron-based matrix are currently under development, and as such, these materials fall outside the scope of this article,
Nonetheless, from our perspective as technicians, the only coating materials we need to concern ourselves with are the ones consisting of carbides and oxides. Put simply, the presence of these coatings on commercially available high-performance brake rotors is what allows high-performance brake to rotors to provide consistent braking performance at brake temperatures in the 4000C to 8000C range, which would destroy non-coated brake rotors in very short order.
Of course, the above assumes that coated brake rotors are used in conjunction with suitable brake friction materials, and it is here where things get complicated. The problem is that friction material formulations are proprietary trade secrets, which means that even if you replace high-performance brake rotors with coated and approved aftermarket replacements, you can never be sure that the new pads you fit will be compatible with the coating on the rotors.
One way around this is to obtain high-performance brake pads only from trusted aftermarket suppliers, but even then, you can never be sure of what you are getting for your customer’s money. The table below describes this problem better than any number of words could-
|
Element |
A / % |
B / % |
C / % |
D / % |
E / % |
|
Carbon |
60.9 |
55.12 |
54.63 |
56.86 |
53.44 |
|
Oxygen |
8.2 |
18.89 |
13.14 |
20.6 |
12.84 |
|
Magnesium |
0.9 |
1.2 |
- |
0.21 |
0.87 |
|
Aluminium |
0.4 |
2.36 |
0.83 |
1.69 |
1.32 |
|
Sulphur |
0.6 |
1.99 |
- |
- |
2.5 |
|
Iron |
25.6 |
20.44 |
27.43 |
16.06 |
20.45 |
|
Barium |
2.9 |
- |
3.97 |
- |
20.45 |
|
Copper |
- |
- |
- |
- |
5.97 |
|
Calcium |
- |
- |
- |
2.16 |
- |
|
Calcium |
- |
- |
- |
2.42 |
- |
Source: https://www.witpress.com/Secure/elibrary/papers/MC05/MC05005FU.pdf
While this table is intended to be purely illustrative, it does show the relative percentages (by weight) of some of the principal ingredients in five sets of commercially available brake pads. The ingredients shown here are typically used as friction modifiers or lubricants in friction materials, which illustrates the point that brake pads are not created equal.
Since similar differences exist in the relative concentrations of binders, fillers, and structural components even in advanced friction materials, any discussion about the advantages or perceived superiority of one brand of brake pad over any other brand is utterly pointless, since nobody knows a), the proportions of all the ingredients in any particular brand of brake pad, and b), whether or not the proportions of ingredients in all aftermarket brake pads are always consistent across production batches.
However, what is known is that many reputable aftermarket manufacturers of friction materials often reverse engineer advanced, OEM-developed friction material formulations. It is not for this writer to express an opinion about the ethical aspects of this practice, but what this writer can say based on his long experience as a master technician, is that successful reverse engineering programs usually yield aftermarket friction materials that are so similar to OEM formulations that the differences are too small to matter even to metallurgists and chemical engineers.
Note though that not all reputable suppliers of aftermarket brake pads will necessarily stock brake pads for all high-performance vehicles- mostly for reasons that involve intellectual property and/or patent rights issues.
We hope that the information provided up to this point has adequately answered the question of why OEM brake parts should be the first choice when performing brake servicing/replacements on high-performance vehicles. However, there is one other issue that needs some discussion, this issue being the practice of-
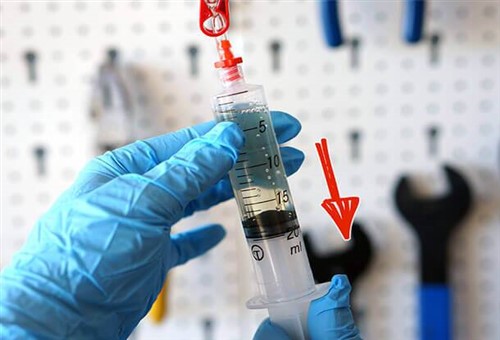
Image source: https://epicbleedsolutions.com/blogs/faq/what-is-meant-by-degassing-brake-fluid
This image shows how "degassing" of brake fluid is supposed to work. In this example of the process, a syringe is partially filled with brake fluid; the syringe is the blocked off, and the plunger is pulled downward to create a low-level vacuum above the brake fluid in the syringe. The theory is that the vacuum will cause all the air or other gases in the brake fluid to coalesce and rise to the top, from where the now-released gases can be vented to the atmosphere. So, is this effective or even necessary?
Common sense dictates that some dissolved air will always be present in brake fluid, even in brake fluid that is kept in sealed metal containers that are impermeable to air. This has been confirmed by various researchers, and while there are no standards anywhere in the world that control/regulate the quality and/or formulation of brake fluid, most brands in use in the Western world contain about 2% dissolved oxygen as the result of manufacturing and packaging processes. Even synthetic brake fluid formulations contain some dissolved oxygen, but as far as this writer is aware, no manufacturer of high-performance vehicles, or any vehicles for that matter, has recommended that this dissolved oxygen be removed from brake fluid before the fluid is used in a vehicle.
In the case of the syringe method, it is clear that the visible bubbles are made of air that is drawn into the syringe past the rubber seal, so what is happening in this example is that additional air and moisture is being drawn into the brake fluid, thus increasing both the water and dissolved oxygen content of the sample. Moreover, since the brake fluid in a master cylinder reservoir is in direct contact with the atmosphere via one or more venting valves in the reservoir cap, there seems to be little point in fiddling with syringes and small amounts of brake fluid to "improve" the brake fluid.
So, since brake fluid (except silicone-based formulations) is hygroscopic, brake fluid in reservoirs will absorb about 3% of its total volume in water over about two years, which means that a freshly-flushed brake system on a high-performance vehicle is extremely unlikely to start experiencing poor braking performance as the result of degraded brake fluid.
We only mention the practice of "degassing" brake fluid because it has the potential to contaminate new brake fluid with excessive amounts of air and water, which is a potentially dangerous thing to do. Therefore, customers need to be educated not only on the dangers of this practice at every opportunity, but also on the need to have the brake fluid in their vehicles replaced at least once every two years, or more often in humid climates, which leaves us with this-
While most owners of high-performance vehicles are not always aware of the specifics of the brake parts their vehicles require for safe operation, long experience has taught that most owners are much less bothered by the high costs involved, than they are about the need for the brakes on their vehicles to be in perfect working order.
Most owners of high-performance vehicles also appreciate a little education and additional effort on our part in this vitally important aspect of the maintenance and care of their vehicles.