
We have all had our fair share of trying to diagnose and resolve weird, strange, and seemingly non-sensical electrical issues on our customer’s vehicles. The experienced technicians among us will recognize these kinds of symptoms; central locking systems that cycle rapidly between “locked and open”, alarms that go off for no apparent reason, gear shift issues on automatics, unexplained, and sometimes, unexplainable misfires, rough running, and even hard starting when the engine cranks normally.
If you are not experienced or have had little exposure to electrical diagnostics, you might be tempted to cast a wide diagnostic net, only to come up empty, with no viable diagnostic paths t follow. In many, if not most cases of strange and/or weird symptoms, the root cause almost always involves the vehicle's battery in one way or another, even though the battery in the affected vehicle may appear new, or relatively new. In a fair number of these kinds of cases this writer has seen, the battery was actually new, but of the wrong type and/or rating, so in this article, we will discuss the advantages, disadvantages, and recommended uses of the various types of automotive batteries in use today. Let's start with the type of battery we all know best, this being
Lead-acid batteries have existed in various forms since before the invention of the motor car, and it was, in fact, only after the first cars were built that some form of standardization in terms of construction, function, and capacity was introduced/adopted by at least some of the major battery manufacturers.
Of course, there have been significant improvements made in lead-acid battery technology in tot 100-odd years since these batteries were first used to power the first electric cars, with the lead alloys used in the plates seeing perhaps the most development and refinement. Nonetheless, despite some new alloys, lead-acid batteries have for all practical purposes, remained virtually unchanged for at least five decades.
There was a simple reason why lead-acid batteries remained unchanged for fifty years or more, chief among them being the fact that there was no need to develop the technology. The electrical systems of the cars of fifty years ago were relatively simple, and the lead-acid batteries of the time were more than capable of providing adequate power for lights, starting, and ignition systems to function effectively and reasonably reliably.
All of this is ancient history, and while the youngest and least experienced among us might find it interesting that the total electrical load on many vehicles was often only about 20 amps. This was true even during spikes, the oldest and most experienced among us might long for the simple lives we led in terms of electrical diagnostics then- even during the chaotic OBD I period. However, things started to change rapidly after 1996, which was when OBD II was first mandated in the US domestic market, and all other major automotive markets, soon after.
OBD II was no doubt good for the environment, but from our perspective as technicians, OBD II’s greatest, and immediate disadvantage was that it greatly complicated the electrical systems of all new vehicles. While we need not delve into these complexities here, we can say that the rapid proliferation of control modules, serial communications systems, and a multitude of sensors demanded a stable power supply to function reliably and effectively- power supplies that the lead-acid batteries and charging systems of the time often could not deliver or sustain reliably.
As might be expected, the battery manufacturing industry responded to this problem by producing lead-acid batteries with progressively bigger capacities, and we soon began seeing lead-acid batteries with 80 and 100 Amp capacities. While adding not only more plates but also thicker plates to lead-acid batteries to increase both their CCA (cold cranking) and RC (reserve) capacities largely resolved the power supply issue, other problems that were (and still are) inherent to lead-acid batteries did not produce a commensurate improvement in durability.
Sadly, limited space precludes a comprehensive discussion on the disadvantages of lead-acid battery technology, but we can say that the two most pertinent issues that affect the durability and performance of lead-acid batteries involve the formation and build-up of sulphurous compounds on the lead plates, and the development and retention of so-called charge memories.
In the case of sulphation, excessive discharging of the battery causes the formation of a sulphurous compound on the lead-alloy plates that prevents effective contact between the plates and the electrolyte, while in the case of charge memories, lead-acid batteries cannot be charged past the upper limit of a "stored" charge memory. This characteristic of lead-acid batteries is the result of extremely complex chemical processes that can begin to occur at almost any point in a lead-acid battery’s life, and while we need not delve into the complexities of these processes here, we can say that lead-acid batteries’ tendency to develop charge memories is arguably their single biggest drawback.
As a practical matter, a charge memory translates into the fact that if a lead-acid battery is operated within a narrow operating range, say between 11 and 10 volts, it eventually becomes impossible to charge that battery to above 11 volts or to discharge the battery to below 10 volts without destroying it. On the upside, though, if such a battery is operated within the limits of its charge memory, it can provide reliable service for extended periods, which brings us to-
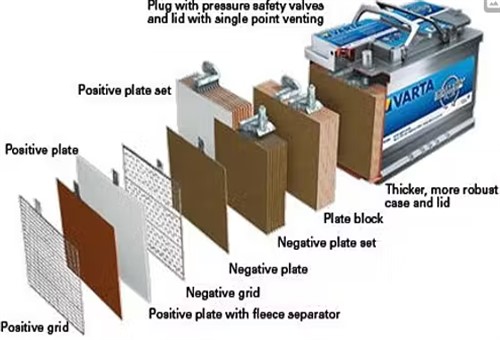
There are many misconceptions about ABM batteries such as the example shown here, including the notion that ABM batteries represent some kind of exotic technology, hence their significantly higher prices as compared to those of simple flooded lead-acid batteries.
The truth is that in their simplest form, AGM batteries are also lead-acid batteries that use largely the same lead alloys and electrolytes as simple flooded batteries do. However, the single biggest differences between AGM and simple flooded batteries involve a) their construction, and b), the performance improvements that come with a different construction. Before we get to the specifics though, it is perhaps worth mentioning that AGM batteries as we know them today were in common use in aviation, mining, and various militaries around the world for many years before the automotive industry noticed their distinct advantages over conventional lead-acid batteries. Let us look at these features in some detail-
Unlike conventional lead-acid batteries that contain only four or five lead-alloy plates per cell, ABM batteries can contain as many as eight to ten (albeit thinner) lead plates per cell. In practice, this effectively doubles the surface area in each cell, and therefore, the electricity generating capacity of each cell.
Moreover, instead of immersing the plates in a liquid electrolyte whose efficiency is greatly reduced by the presence of a large amount of water, the electrolyte in an AGM battery is concentrated in a kind of gel*and absorbed into fibreglass mats that are squeezed into the spaces between the plates. This arrangement guarantees the maximum amount of contact between the plates and the electrolyte, which in turn, ensures that equal amounts of energy is created over the entire surface area of each lead plate in each cell in the battery.
*NOTE: This is not the same as a proper gel battery, in which sand (silica) is used to turn the electrolyte into a thick paste that has the consistency of half-molten peanut butter. Note also that while gel batteries do have their uses, these uses typically do not include use in automotive applications, since gel batteries are very easily damaged in high current-draw situations, such as might occur during prolonged engine cranking.
Nonetheless, since water plays a critical role in the re-absorption of some of the gases that are generated when a battery is not at rest, the electrolyte gel does contain a small percentage of water. However, since the generation of electricity generated both gases and heat, which causes some water to evaporate, AGM batteries are fitted with safety valves in each cell that keeps the water content of the electrolyte under pressure to reduce or eliminate evaporation. This feature makes AGM batteries maintenance free, in the sense that the electrolyte level never needs topping up.
While some conventional lead-acid batteries are also sealed and can be said to be maintenance free, sealed lead-acid batteries can still sulphate or develop charge memories, which typically does not happen with AGM batteries.
In terms of performance, AGM batteries can provide the same short, intense bursts of energy that flooded lead-acid batteries can during engine starting. However, since the energy production cycles in AGM batteries are more stable because their chemistry is kept in (almost perfect) balance by the pressure valves in each cell, their power output is almost devoid of spikes, dips, and ripples that occur as the result of sloshing liquid electrolyte and/or the formation of gas bubbles on the lead plates under some operating conditions.
One other major advantage of AGM batteries over flooded batteries is that they can be fairly deeply discharged, although AM batteries are not true deep-cycle batteries. Nonetheless, AGM batteries can be discharged to about 50 per cent of their capacities for several hundred cycles without suffering adverse effects, which allows their use in vehicles with stop-start functionality. For this reason, AGM batteries in vehicles with stop-go systems should always be replaced with a similarly rated AGM battery. This is because a simple flooded lead-acid battery cannot supply sufficient starting current for the same number of starting cycles/events as an AGM battery can, especially if the simple flooded lead-acid battery also has to power systems like A/C blower fans, headlights, and all required interior lighting.
However, nothing in life is ever perfect, and in the case of AGM batteries, their single biggest disadvantage is that they require specialized charging equipment. As a practical matter, AGM batteries are extremely susceptible to damage caused by even marginal over-charging, and/or undercharging, which means that equipment intended for use on other types of batteries to charge an AGM battery is not suitable for use with AGM batteries.
Below is a generic AGM battery charging profile-
|
Charge Stage |
State of Charge |
Charging Voltage |
Charging Rate |
|
Bulk stage |
0 – 80 per cent |
13V – 14.5V |
0.1C* |
|
Absorption stage |
80 – 100 per cent |
14.2V – 14.9V |
Declining |
|
Float Stage |
100 per cent |
13.2V – 13.8V |
Very low |
* In this context, “C” denotes the charging current. In this example, 0.1C indicates a charging current of 10 per cent of the battery’s amperage rating. For example, if the AGM battery is rated at 60 Amps, 0.1C would be 6 Amps, and if the battery is rated at 100 Amps, 0.1C would be 10 Amps. Note that while higher charging currents can be employed to reduce charging times, higher charging currents always reduce the battery’s useful life.
Note that the 14.9V charging voltage shown during the absorption stage in this example is just that, an example. Some batteries require voltages (during this stage) that can be as high as 15.5V to 16.0V, with the actual required voltage depending on the battery's brand and rating, as well as its overall state of health. Thus, the only way to be sure you are charging an AGM battery correctly is to use charging equipment that is specifically designed to charge this type of battery.
The heart of these chargers is a microprocessor that can “read” all of the design parameters of these batteries, and automatically adapt charging profiles to suit individual batteries, as opposed to offering a one-size-fits-all approach to a certain type of battery-as is the case with the simple analogue chargers most of us still use to charge conventional flooded lead-acid batteries.
It is perhaps worth mentioning that while AGM batteries have greatly reduced electronics failures on modern vehicles, this added reliability comes at the price of the greatly increased complexity of modern charging systems. For instance, many modern vehicles come with battery monitoring systems, changing system control modules and variable alternators, all of which require that new AGM batteries be integrated into the vehicles' electrical system to ensure reliable operation not only of the battery but also of the larger charging system and its control mechanisms, which brings us to-

In terms of their operating principles, there are no significant differences between how the lithium-ion batteries in our phones, cameras, laptop computers, and tablets work and how the lithium-ion batteries in electrical and hybrid vehicles work. In fact, lithium-ion batteries were originally invented and developed for application in various consumer electronic markets long before car manufacturers began to realize the major advantages of lithium-ion technology over nickel-cadmium and nickel-metal hydride batteries used in early hybrids and electric cars.
In their simplest form, lithium-ion batteries also generate electricity through the flow of ions between an electrode and a cathode via an external circuit. However, while lead-acid batteries use lead ions flowing through a simple acid and water solution, lithium-ion batteries use one of several types of lithium ions and an electrolyte that typically consists of an organic salt, such as LiPF6 that is diluted into one of several solvents. These include, but are not limited to ethylene carbonate, diethyl carbonate, dimethyl carbonate, and ethyl carbonate. In addition, the power delivery characteristics and storage capacity of the battery are determined by the addition of one or more additives that prevent a) the formation of dendritic substances on the plates* and b) the general degradation of the electrolyte solution.
* This is roughly analogous to the formation of sulphurous compounds on the lead plates in lead-acid batteries. However, while sulphation reduces energy production, dendrites are "spikes" of corrosion that can intrude into the electrolyte, thus causing fatal short circuits.
Moreover, apart from the specific formulation of the electrolytic solution, the materials used in the construction of the cathodes and electrodes, as well as the amounts of precious metals, such as cobalt and nickel, used in the internal circuitry and plates of the battery all play critical roles in both the power density and power ratio of any given lithium-based battery.
As a practical matter, the various formulations of metals and electrolytes all produce slightly different chemical actions/reactions, which makes it possible to “fine-tune” a given lithium-ion battery design to operate at a lower nominal voltage, while delivering roughly the same power density as a battery that operates at a higher nominal voltage. For example, some electric vehicles operate at about 200V, while others operate at 400V and more, while the dimensions of the overall dimensions of battery packs are roughly the same.
Even though some advanced modern lithium-ion batteries can now be charged in under an hour with some categories of charging equipment, the single biggest drawback of these batteries is the fact that the overall assembly of a battery pack consists of dozens of small, low-voltage cells that are all connected to each other in various ways and patterns. This necessarily requires a system that can monitor each individual cell's temperature as a direct function of its state of health, as well as its rate of charge/discharge. Such differences are typically reported as trouble codes that indicate imbalances in the battery, and while such codes are useful diagnostic aids, establishing the root cause of such an imbalance is not easy.
For instance, if the issue is caused by loose or bad connections between cells, determining which particular connections are at fault requires specialized equipment and training. Similarly, if the issue is caused by one or more bad and/or damaged cells, identifying the affected cells also requires specialized equipment and training.Nonetheless, modern lithium-ion batteries are fairly robust and reliable, meaning that battery imbalance issues are somewhat rare. Therefore, most lithium-ion batteries in hybrid and electric vehicles offer power densities that are between 300 and 500 hundred times higher than even the most advanced lead-acid flooded batteries.
It is perhaps worth noting the difference between power density and capacity. Here are some details-
Capacity
This refers to the amount of energy any given battery can store, and in the automotive industry, this value is always expressed as kilowatt-hours, or as kWh, for short. In the case of lithium-ion batteries, this value is determined by the number of cells in the pack, how they are arranged and connected, as well as by the formulation of the electrolyte and the materials/metals used in the battery’s construction.
Power density
This value is simply the ratio between the amount of energy stored in the battery, and its overall size and weight. As mentioned elsewhere, most lithium-ion batteries in automotive applications today offer power densities of between 300 and 500 W/kg, which is at least ten times higher than that offered by conventional flooded lead-acid batteries.
A simple analogy with a water tank might make it easier to appreciate these concepts: think of energy density as being equal to the size/volume of the tank, and power density being equal to the time required to drain the tank as quickly as possible through an existing outlet, which leaves us with this-
As matters stand now, lithium-ion batteries in their current form represent the pinnacle of automotive battery technology, but several new developments and technologies are now being developed. For instance, several carmakers, including Ford and Nissan, are now working on developing batteries that use solid electrolytes for use in hybrid and electric vehicles, with Nissan preparing to launch such a battery in 2025.
Other advances now under development include structural batteries, which are intended to form parts of the structures they are powering. One example of this might be body panels on light vehicles or chassis beams on trucks that are actually batteries, but these kinds of batteries are unlikely to see the light of day for at least another decade- or at least until car manufacturers have worked out how to mass-produce reliable load-bearing batteries.
Until these advanced forms of batteries arrive, however, the best thing we can do is to understand the capabilities, intended uses, and most importantly, the limitations of the batteries we now have. Having at least a working knowledge of what batteries are supposed to do, as opposed to what we sometimes expect them to do, goes a long way towards explaining some of the weird and strange electrical symptoms and system failures we sometimes see, so we hope this article has provided some new insights into this field of diagnostics.